Background
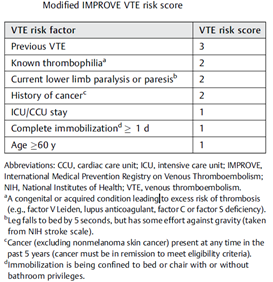
The practice of providing thromboprophylaxis on discharge from hospital in the pre-Covid era is not guideline recommended1 based on several trials looking at various NOACs in medically ill patients2–4. Though the incidence of thrombosis is reduced, mortality does not show any significant change. The major adverse effect is the increase in severe bleeding. To get around this, scores like the Modified IMPROVE VTE Risk Score have been utilised to select patients at higher risk of thrombosis in an effort to identify the population who would benefit from such prophylaxis5. This strategy still awaits RCT confirmation.
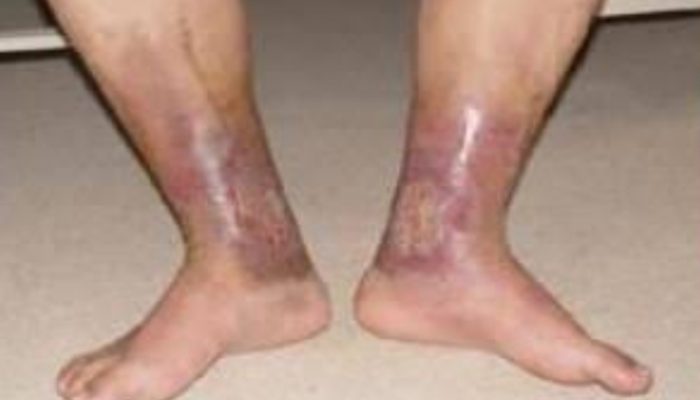
Incidence of post-discharge thrombosis in Covid-19
The incidence of post-discharge thrombosis in Covid-19 cohorts have shown a varying incidence ranging from 0.48%6 to 2.6%7,8 . Current data do not confirm an increased risk compared to non-Covid patients when tested as outpatients or post-discharge9.In addition, we need to factor in the lower incidence of thromboses in South Asians.
Trials of post-discharge thromboprophylaxis in Covid-19
There are no published trials of thromboprophylaxis in Covid-19 but several are in the pipeline10,11. A recently published registry of post-discharge thromboprophylaxis suggested that the small number of patients discharged on thromboprophylaxis had better outcomes (but not improvement in all-cause mortality)12. Due to the inherent shortcomings of registry data this study is not robust enough to change practice but a reminder, as the authors recommend, to perform RCTs.
D-Dimer
There is no evidence to suggest standalone D-Dimer testing is of any value in deciding on post-exposure prophylaxis. A series of 150 patients with Covid had a 25% incidence of raised D-Dimers at 6 weeks after diagnosis with 8% having elevations above twice the upper limit of normal. One in five of the patients with raised D-Dimers had CTPAs at some point which were negative. There were no fatalities13.
Guidelines
The NIH guidelines state14 “VTE prophylaxis after hospital discharge is not recommended for patients with COVID-19 . For certain high-VTE risk patients without COVID-19, post-discharge prophylaxis has been shown to be beneficial. The Food and Drug Administration approved the use of rivaroxaban 10 mg daily for 31 to 39 days in these patients. Inclusion criteria for the trials that studied post-discharge VTE prophylaxis included:
- Modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) VTE risk score ≥4; or
- Modified IMPROVE VTE risk score ≥2 and D-dimer level >2 times the upper limit of normal.
Any decision to use post-discharge VTE prophylaxis for patients with COVID-19 should include consideration of the individual patient’s risk factors for VTE, including reduced mobility, bleeding risks, and feasibility. Participation in clinical trials is encouraged.”
The ACCP guidelines state15 “In patients with COVID-19, we recommend inpatient thromboprophylaxis only over inpatient plus extended thromboprophylaxis after hospital discharge.….extended thromboprophylaxis would result in a net benefit in patients with COVID-19 at low bleeding risk, if the risk of symptomatic VTE would be above 1.8% at 35 to 42 days after hospital discharge.”
Summary
- Incidence of post-discharge venous thrombosis after Covid appears to be similar to other acute illnesses.
- Routine post-discharge thromboprophylaxis is not recommended in Covid-19 but may be used in select cases with high risk of thrombosis and low risk of haemorrhage using validated scores.
- D-Dimer testing by itself is not recommended to decide on post-discharge thromboprophylaxis.
Randomized controlled trial results are expected soon to further inform opinion.
31/05/2021
- Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
- Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus Enoxaparin for Thromboprophylaxis in Medically Ill Patients. N Engl J Med. 2011;365(23):2167-2177. doi:10.1056/NEJMoa1110899
- Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for Thromboprophylaxis in Acutely Ill Medical Patients. N Engl J Med. Published online February 6, 2013. doi:10.1056/NEJMoa1111096
- Spyropoulos AC, Ageno W, Albers GW, et al. Rivaroxaban for Thromboprophylaxis after Hospitalization for Medical Illness. N Engl J Med. 2018;379(12):1118-1127. doi:10.1056/NEJMoa1805090
- Spyropoulos AC, Lipardi C, Xu J, et al. Modified IMPROVE VTE Risk Score and Elevated D-Dimer Identify a High Venous Thromboembolism Risk in Acutely Ill Medical Population for Extended Thromboprophylaxis. TH Open. 2020;04(1):e59-e65. doi:10.1055/s-0040-1705137
- Roberts LN, Whyte MB, Georgiou L, et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136(11):1347-1350. doi:10.1182/blood.2020008086
- Salisbury R, Iotchkova V, Jaafar S, et al. Incidence of symptomatic, image-confirmed venous thromboembolism following hospitalization for COVID-19 with 90-day follow-up. Blood Adv. 2020;4(24):6230-6239. doi:10.1182/bloodadvances.2020003349
- Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342-1346. doi:10.1182/blood.2020007938
- Roubinian NH, Dusendang JR, Mark DG, et al. Incidence of 30-Day Venous Thromboembolism in Adults Tested for SARS-CoV-2 Infection in an Integrated Health Care System in Northern California. JAMA Intern Med. Published online April 5, 2021. doi:10.1001/jamainternmed.2021.0488
- Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent Randomized Trials of Antithrombotic Therapy for Patients With COVID-19. J Am Coll Cardiol. 2021;77(15):1903-1921. doi:10.1016/j.jacc.2021.02.035
- Leentjens J, Haaps TF van, Wessels PF, Schutgens REG, Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents—lessons after 1 year. Lancet Haematol. 2021;0(0). doi:10.1016/S2352-3026(21)00105-8
- Giannis D, Allen S, Tsang J, et al. Post-Discharge Thromboembolic Outcomes and Mortality of Hospitalized COVID-19 Patients: The CORE-19 Registry. Blood. Published online 2021. doi:10.1182/blood.2020010529
- Townsend L, Fogarty H, Dyer A, et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. 2021;19(4):1064-1070. doi:https://doi.org/10.1111/jth.15267
- NIH. Antithrombotic Therapy. COVID-19 Treatment Guidelines. Published February 2021. Accessed May 26, 2021. https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/
- Moores LK, Tritschler T, Brosnahan S, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. CHEST. 2020;158(3):1143-1163. doi:10.1016/j.chest.2020.05.559
High Risk of Bleeding
Active cancer at randomization,
Dual antiplatelet therapy at baseline,
History of bronchiectasis/pulmonary cavitation,
Active gastroduodenal ulcer
Any bleeding in the previous 3 months